Beyond IEC 60601: Standards and Medical Device Safety by Design
Posted October 29, 2024 by Conor Duffy
Modern Strategies for Ensuring Electrical Safety in Healthcare Devices
Key Takeaways:
- Strict Safety Standards: Medical diagnostic and surgical systems must adhere to stringent safety standards, with IEC 60601 being the most well-known. Understanding these standards and the influence of regulatory bodies like the FDA and EU is crucial for global compliance.
- Market Growth: The global medical device market is rapidly expanding, projected to grow from $471 billion in 2020 to $623 billion by 2026. This growth necessitates evolving safety standards to balance technological advancement with safety.
- Historical Context: Safety standards like IEC 60601 have evolved from earlier regulations, reflecting the medical industry’s proactive approach to safety and adaptation to modern device functionalities.
- IEC 60601 Framework: This series of standards focuses on mitigating risks such as electric shock, mechanical hazards, and radiation. It also addresses labeling, documentation, and software aspects of medical devices.
- Complementary Standards: Other standards, such as ANSI/AAMI and ISO 14971, complement IEC 60601 by providing additional safety perspectives and risk management processes.
- Regulatory Oversight: The FDA and EU play key roles in ensuring medical device safety through rigorous testing and compliance processes. Despite differences, global harmonization efforts aim to streamline compliance.
- Supplier Support: Manufacturers rely on suppliers for products that simplify compliance. Advanced Energy, for example, offers power solutions that meet IEC 60601-1 standards and operates Customer Experience Centers to support equipment designers.
- These points highlight the importance of safety standards in the medical device industry and the ongoing efforts to ensure compliance and safety in a rapidly growing market
Medical diagnostic and surgical systems must follow some of the world’s strictest safety standards. The best known is the International Electrotechnical Commission (IEC) 60601, which ensures there is no risk to patients and/or operators, but there are others to consider when developing healthcare products for worldwide markets. Understanding these standards and the influence of international regulatory bodies such as the Food and Drug Administration (FDA) and the European Union (EU) (for CE marking) are key to global compliance. Here we consider the issue of medical safety, look at the situation to date and provide a glimpse into the future of relevant safety standards and their potential impact.
According to the Facts and Factors market research report, the global medical device market is rapidly growing, expanding at a CAGR of 5% from $471 billion in 2020 to an anticipated $623 billion by 2026. Set against this backdrop of expansion and innovation, the industry faces the challenge of balancing the drive for technological advancement with the imperative of ensuring the highest level of safety for patients and healthcare providers. This creates a continual need for evolving safety standards.
Such standards are nothing new – IEC 60601 itself is the latest iteration of rules and regulations that can trace their roots back to the National Electrical Code in 1897 and significant milestones like the development of Ground Fault Circuit Interrupters in the 1960s and the establishment of the NFPA 70E standards in 1976. This history shows the medical industry’s proactive stance in addressing basic safety concerns and adapting to the advanced functionalities of modern medical devices.
Deep Dive into Electrical Safety Standards: Beyond IEC 60601
The IEC 60601 series is a fundamental framework for electrical safety in medical devices and includes a set of international standards designed for the safety and essential performance of medical equipment. It covers general requirements for basic safety and essential performance (IEC 60601-1) along with numerous standards for specific types of equipment (IEC 60601-2-XX series).
IEC 60601 focuses mainly on mitigating risks related to electric shock, mechanical hazards, radiation and excessive temperatures. This standard also addresses the need for adequate labeling and documentation to enhance safety. What’s more, it considers the software and system aspects of medical devices, which are increasingly integral in the era of digital healthcare.
However, the scope of electrical safety in medical devices isn't limited to IEC 60601. Other standards play vital roles and often interact with the IEC 60601 series. Among these are the American National Standards Institute/Association for the Advancement of Medical Instrumentation (ANSI/AAMI) standards that often provide a more U.S.-focused perspective on medical device safety, complementing the international viewpoint of the IEC. For example, ANSI/AAMI ES60601-1 is the U.S. national version of the IEC 60601-1 standard, adapted to meet specific U.S. regulatory requirements.
In addition, International Organization for Standardization (ISO) standards, such as ISO 14971, which deals with the application of risk management to medical devices, offer a broader approach to safety that encompasses electrical aspects and the overall lifecycle risks of medical devices. This standard encourages manufacturers to establish a risk management process, identifying and evaluating risks associated with their devices, and acting appropriately to mitigate them.
Regulatory Bodies and International Compliance
Global regulatory oversight also adds to the complexities of electrical safety standards such as IEC 60601.
The FDA's stringent regulatory process, for example, targets the safety and efficacy of medical devices before they enter the U.S. market. This involves comprehensive pre-market testing, approval and continuous post-market surveillance. Meanwhile in Europe, CE marking signifies a product's compliance with EU health, safety and environmental requirements. This includes adherence to the Medical Devices Regulation (MDR), with the goal that medical devices sold in the European Economic Area meet essential safety standards.
While they share the same commitment to safety and efficacy, the CE marking process and the FDA's approach to medical device regulation differ in several important aspects:
| FDA | CE Marketing | |
| Approval Process | Requires a more rigorous pre-market approval process for certain classes of devices, which often includes detailed clinical trial data. | Generally, focuses on demonstrating compliance with the relevant European directive or regulation, which can be a less stringent process compared to the FDA's pre-market approval, especially for lower risk devices. |
| Clinical Data Requirements | Typically demands extensive clinical data for higher-risk devices, and this data must often be derived from clinical trials conducted in the U.S. | The requirement for clinical data is less stringent, and there is greater flexibility in the types of clinical evidence that can be used to support device safety and efficacy. |
| Quality System Regulation | Enforces Quality System Regulation (QSR), which requires manufacturers to follow specific procedures and documentation for design, manufacturing, and quality assurance. | Requires compliance with ISO 13485 standards for medical devices, which have a similar focus but differ in terms of specific procedures and documentation. |
| Post-Market Surveillance | Has a robust post-market surveillance system, including mandatory reporting of adverse events and periodic inspections. | Also requires post-market surveillance, but the mechanisms and frequency of reporting can differ, with a recent emphasis on more rigorous surveillance under the new MDR. |
| Scope and Enforcement | Its regulations are legally binding in the United States, and the FDA has enforcement authority including the ability to recall non-compliant products. | The CE mark indicates conformity with EU standards, which are enforced by individual member states and require manufacturers to self-declare their compliance with these standards. |
| Classification System | Uses a classification system based on the risk associated with the device (Class I, II, and III). | Also classifies devices based on risk, but the criteria and categories can differ from those used by the FDA. |
| Documentation and Registration | Requires a formal submission process for most devices, which can include a Pre-market Approval (PMA) or 510(k) submission. | Manufacturers must compile a technical file or design dossier demonstrating compliance with the applicable directive. This documentation is usually reviewed by a notified body, except for lower risk devices, which may only require self-certification. |
Distinctions between medical device safety standards in the U.S. and Europe illustrate a varied regulatory landscape. While the core objective to safeguard public health is universal, each region has its own idea of how to reach this goal.
Regulatory harmonization and global compliance are achieved through several key strategies such as international standard development, collaborative regulatory forums, mutual recognition agreements, continuous monitoring and updates, guidance documents and other collaborative efforts. However, challenges remain due to differences in national healthcare systems, regulatory philosophies and legal frameworks.
How Suppliers Can Help
The complexities of addressing the various certification requirements for globally deployed medical equipment means that manufacturers increasingly expect their suppliers to provide products that simplify compliance and offer guidance, technical support and even pre-certification testing.
Regulatory harmonization and global compliance are achieved through several key strategies such as international standard development, collaborative regulatory forums, mutual recognition agreements, continuous monitoring and updates, guidance documents and other collaborative efforts. However, challenges remain due to differences in national healthcare systems, regulatory philosophies and legal frameworks.
How Suppliers Can Help
The complexities of addressing the various certification requirements for globally deployed medical equipment means that manufacturers increasingly expect their suppliers to provide products that simplify compliance and offer guidance, technical support and even pre-certification testing.
Recognizing this, Advanced Energy provides standard, modular and custom power delivery and control solutions that make certification easier. Most recently, Advanced Energy launched the world’s first convection cooled CF rated Power Platform, SL Power NCF150 series of high-isolation, low-leakage current AC-DC power supplies that enable medical equipment designers to meet the CF requirements of the IEC 60601-1 medical safety standard using off-the-shelf products. With the launch of the new product, Advanced Energy now offers standard, compact products that combine the performance and reliability demanded by complex medical systems with the high isolation voltage and low leakage current essential for CF certification.
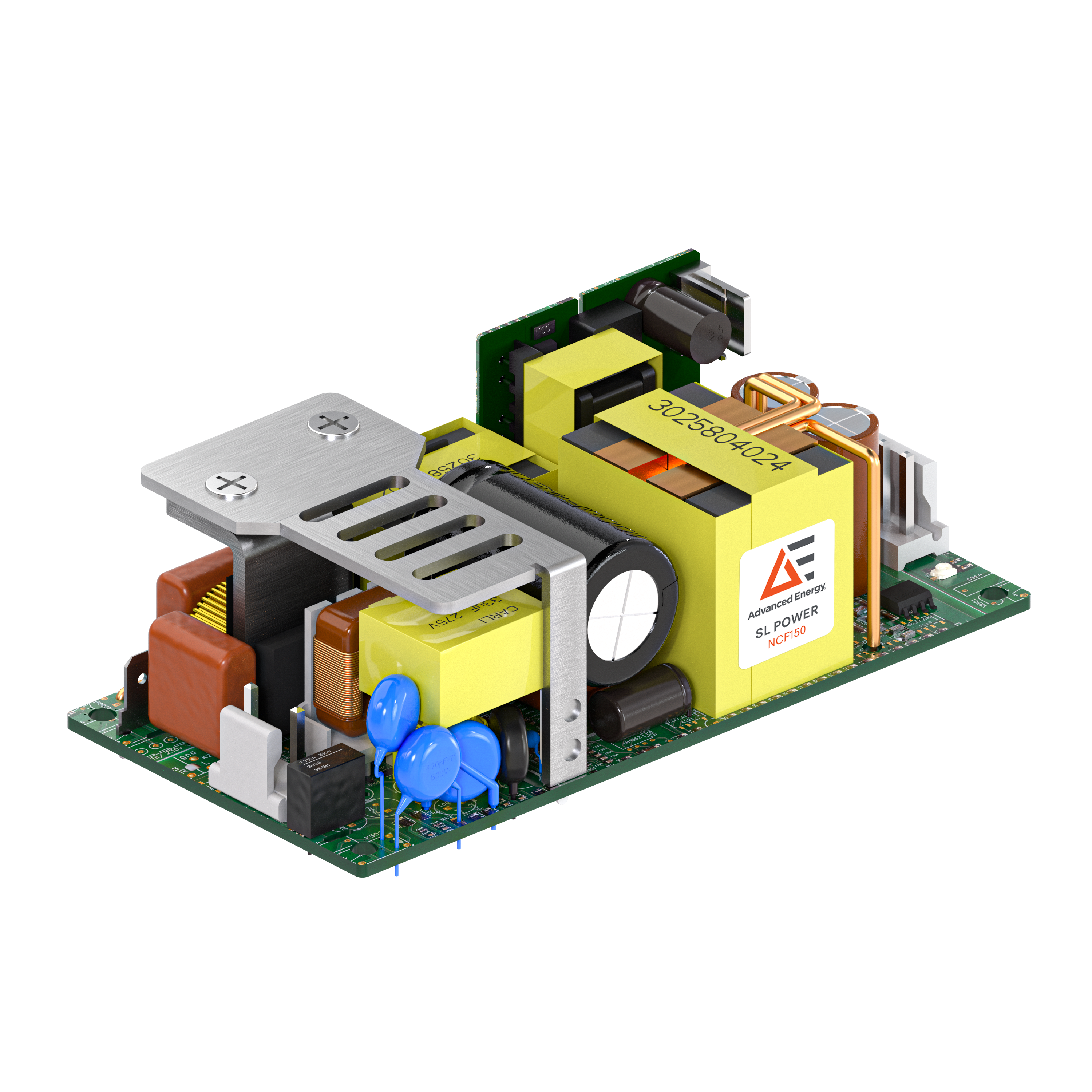.png) Fig. 1. Advanced Energy’s new off-the-shelf, CF-rated NCF 150 series of power supplies.
Fig. 1. Advanced Energy’s new off-the-shelf, CF-rated NCF 150 series of power supplies.
In addition, Advanced Energy operates Customer Experience Centers (CECs) in strategic locations worldwide that are staffed by engineers who work with customers at the system level. These centers help to guide equipment designers through the development journey to minimize risk and ensure that all performance, safety and regulatory targets are met in designs that are cost-optimized for target applications. Click here for more information on Advanced Energy’s CECs.
Conor Duffy
Advanced Energy
Conor Duffy has more than 20 years of experience across General Management, Sales, Product Management & Technical Marketing roles in the in the Power Supply and Industrial Electronics industries. He joined Advanced Energy in 2017 as part of the acquisition of Excelsys Technologies where he was then CEO. Conor holds an MSc. in Technology Management from Smurfit Business School, University College Dublin, a BSc. Electrical & Electronic Engineering from University College Cork and has also completed Executive Leadership Programs at IESE Business School, Barcelona and IMD in Lausanne.
More posts by Conor Duffy

