Medical Power Supplies
Most designers nowadays elect to use standard commercially available medical grade power supplies, or seek a customized medical PSU. Using power supplies that meet medical requirements, and are already pre-approved to the EN60601-1 safety standard, helps medical device and system manufacturers speed compliance testing of products. Medical OEMs can also use medically approved power supplies to minimize the risk of encountering unexpected development problems outside their own area of expertise, which might negatively impact launch targets.
Medical Applications
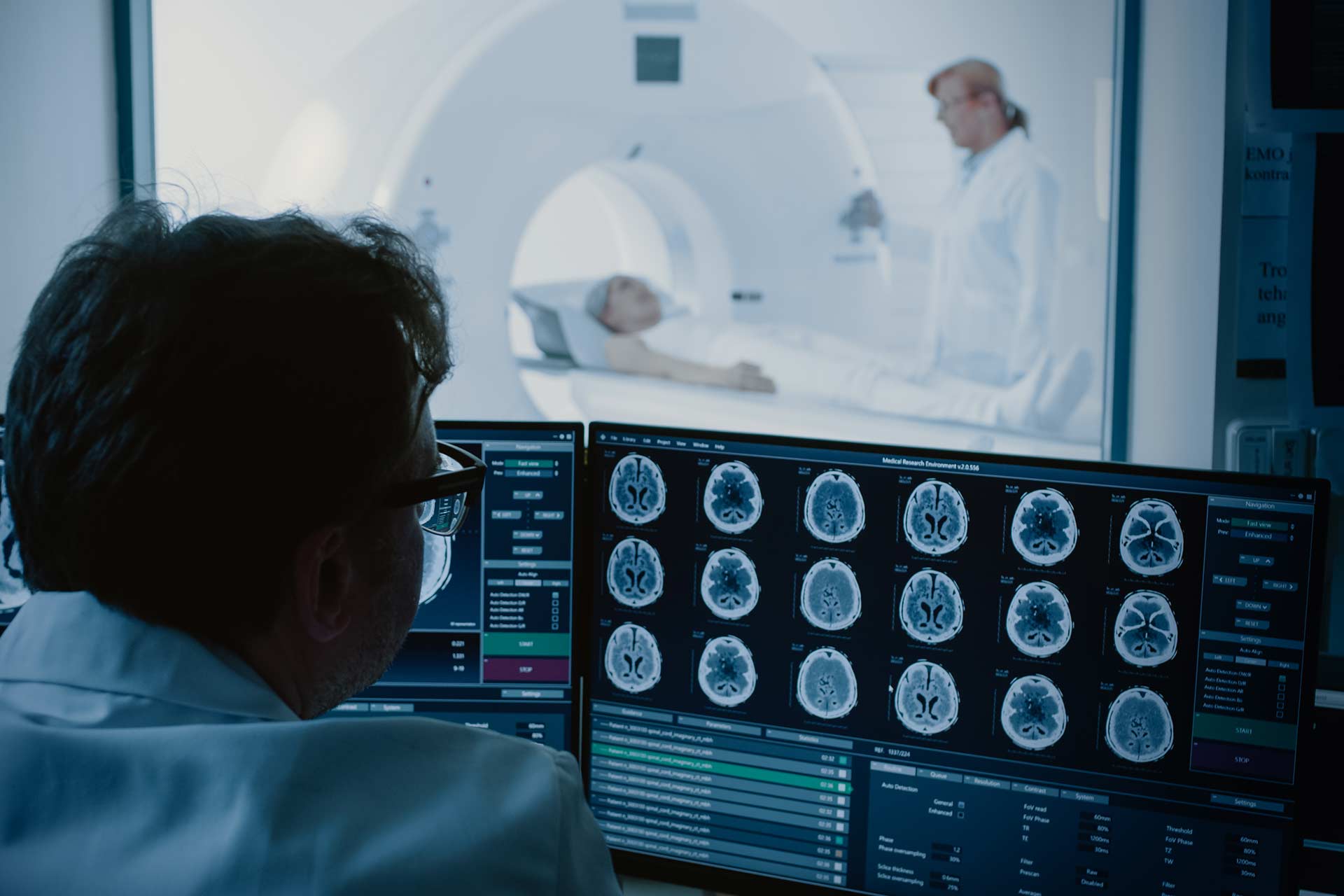
Medical Imaging Power Supplies
We design power supplies that ensure high stability and low ripple to deliver accuracy, resolution, and repeatability for medical imaging.
Life Science
Advanced Energy's power supplies offer a reliable, accurate, ultra-low ripple high voltage power delivery that elevates the performance of life science instruments.
Electrosurgery
Advanced Energy’s compact high and low voltage power supplies allow precise control while offering best-in-class power density and reliability for electrosurgery.
Medical Lasers
We design power supplies for demanding medical and cosmetic laser applications that offer high performance, reliability, and compliance.
Patient Care
Our standard and modified power supplies meet the electrical specifications, ruggedness, and reliability necessary for patient care applications.
Featured Products
Frequently Asked Questions
Featured Resources

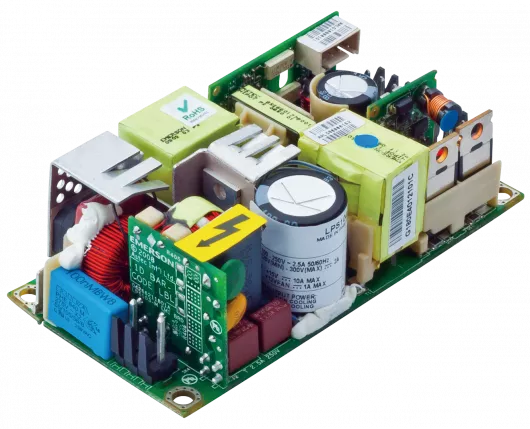
FCM 30K Data Sheet
Download Evergreen Vento FCM 30K Datasheet to discover how AE’s 30kW AC-DC power supply with smart fan control and high efficiency meets the needs of industrial and medical embedded power applications.
Medical Equipment Power Supplies Checklist

Medical Power Supplies Brochure
See our Medical Power Supplies Brochure to discover how AE broad medical portfolio meets your power supply needs.

Low Voltage Medical Power Supplies Brochure
See our Low Voltage Catalog to discover how AE Low Voltage powers your medical power supplies.

UltraVolt High Voltage Quick Select Chart
View UltraVolt high voltage power supplies' technical specifications and easily select the product right for you.

Testing Power Supplies for Medical Electronics Applications White Paper

Sports injury recovery station with hot and cold thermals pads and applied pressure

Surgical Robotics Selector Guide
Surgical Robotics Selector Guide

Medical Power Supply Selector Guide
Explore AE's broad medical supplies portfolio by downloading Medical Power Supply Selector Guide.

High Voltage Power Supply required for use in intraoperative EMG monitor (nerve integrity monitoring system)

SL Power External Power Supplies Selector Guide
Download SL Power External Selector Guide to explore AE’s external power supply portfolio, with visual comparisons and technical specs to help you choose the right solution for your application.

SL Power Internal Power Supplies Selector Guide
Download SL Power Internal Selector Guide to compare AE’s internal power supply offerings, featuring key specifications and visuals to guide your selection process for embedded power needs

Configurable power supply is preferred solution for dedicated imaging equipment
Download Configurable Power Supply is Preferred Solution for Dedicated Imaging Equipment Case Study to gain insights on how AE’s Excelsys Ultimod PSU delivers low-noise, multi-output power for cardiac SPECT systems with medical safety compliance.

System Power Selector Guide

Customer Problem Solved by CEC Team and Modified Power Supply

Ensuring Safety Compliance for a Medical Device Manufacturer
Technical Videos














.png?resizemode=force&maxsidesize=128)









.jpg?resizemode=force&maxsidesize=500)
.jpg?resizemode=force&maxsidesize=500)